Individualizing Prostate Cancer Immunotherapy by a Mathematical Model
Background: Prostate cancer (PCa) is the second most common malignancy in men. Efforts to target PCa by cell-based immunotherapy, aimed at boosting specific anticancer immune responses, are being recognized as promising approaches. Results of a recent phase II clinical trial applying whole-cell vaccination have been encouraging, but have lead only to partial responses in a fraction of PCa patients. Considering the complexity of the immune system-tumor interplay in PCa, application of a simple mathematical model could significantly enhance the efficacy of PCa vaccination in individual patients.
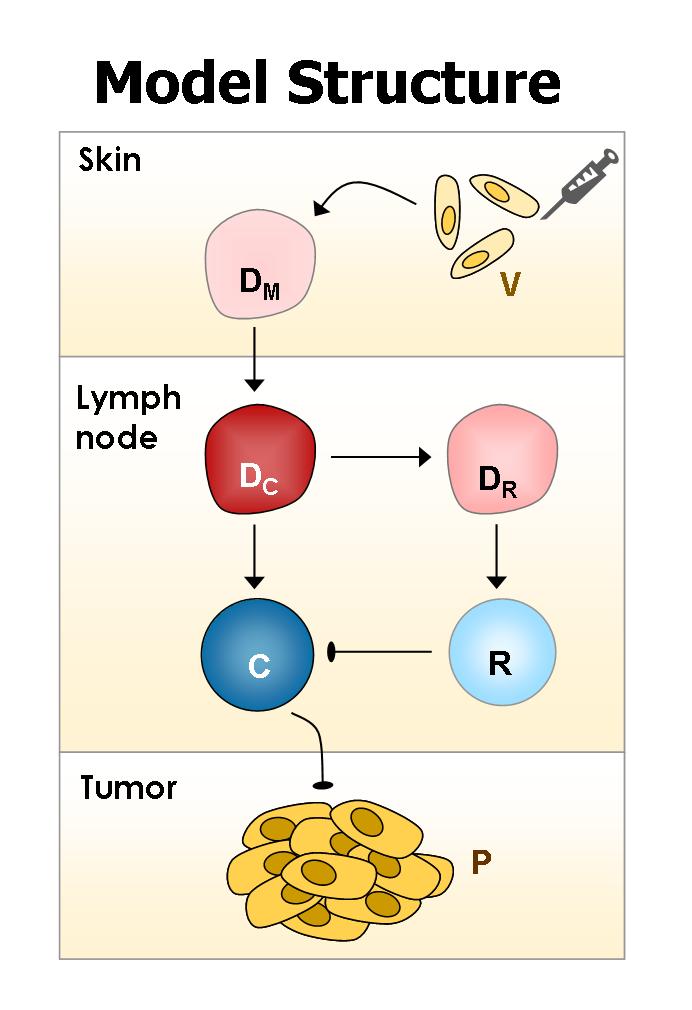
Methods and Results: We have developed a mathematical model that encompasses basic interactions of the vaccine, immune cells and cancer cells (Figure 1). Data obtained from published preclinical and laboratory experiments were used for estimation of most model parameters. The model was successfully validated using clinical data from a phase 2 trial testing an allogeneic PCa whole–cell vaccine (Michael, et al., 2005). We evaluated selected patient–specific parameters, using “training data” consisting of prostate–specific antigen (PSA) levels recorded prior to and during the early stages of vaccination treatment in each of the phase-2 trial patients. Subsequently, the model was simulated to yield patient–specific PSA dynamics in the later stages of therapy, and compared these predictions with the clinically measured PSA values (“validation data”). By using only nine to 15 initial PSA measurements (six to ten of which were initial in–treatment values) for training, we arrived at a model that accurately and robustly described the entire course of PSA dynamics in the 12 of 15 vaccination–responsive patients (R2=0.972). Interestingly, we discovered that the number of training data required for validation differed among patients, emphasizing the importance of patient–specific analysis.
Each individually validated model was then used to suggest alternative vaccination regimens to optimize therapeutic effects. By simulating numerous alternative treatment protocols, we could single out personalized protocols that could enhance therapeutic efficacy for most patients. Model-recommended, clinically feasible vaccination regimens include doses of between 3.6-6.7 x108 cells, which are to be applied at inter-vaccination intervals of 1-21 days.
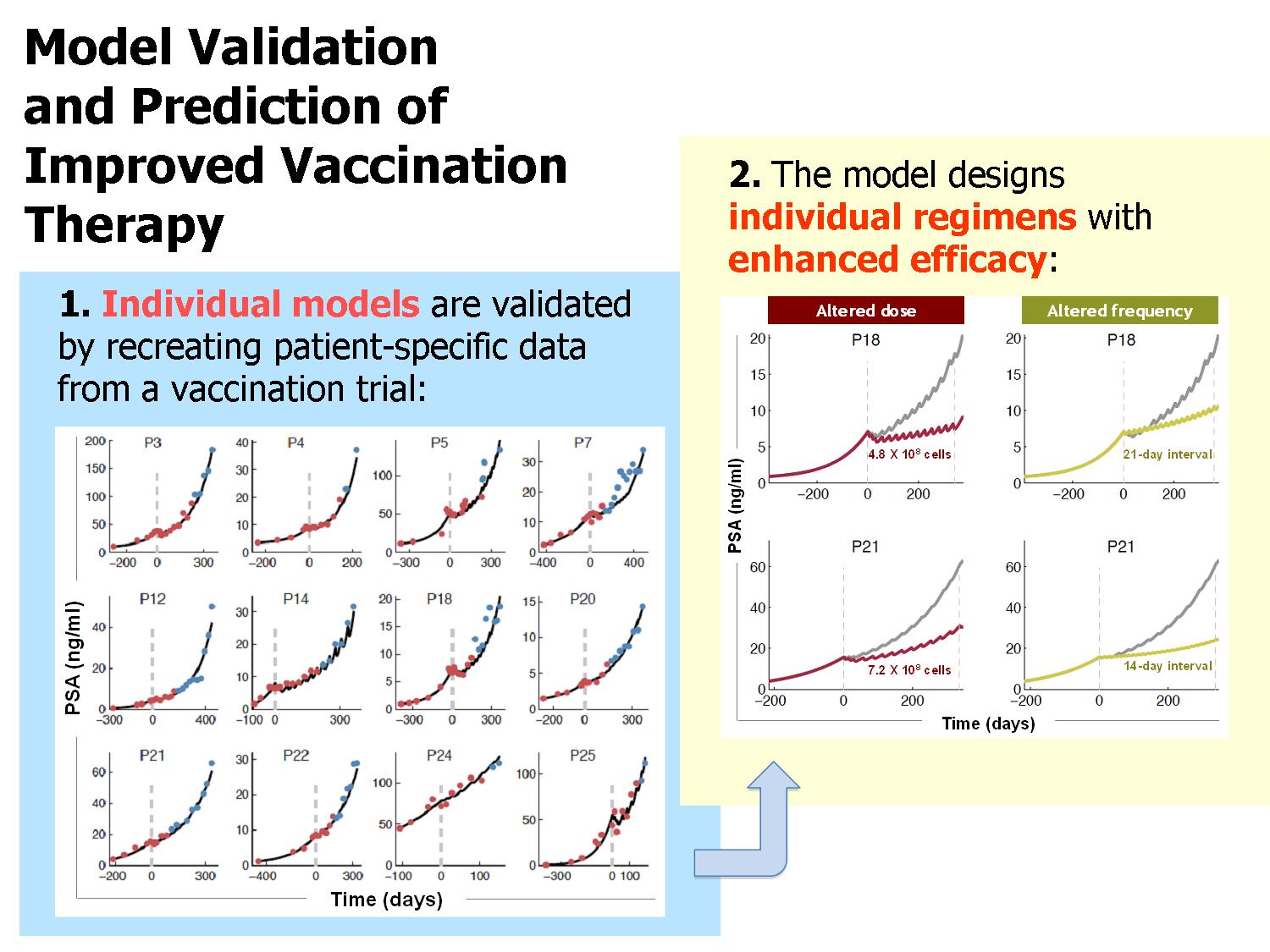
Conclusions: In summary, a small number of pretreatment and initial in–treatment PSA measurements are sufficient for constructing patient–specific PCa immunotherapy models, which accurately predicted the subsequent PSA dynamics. While PSA levels are of limited predictive value themselves, their analysis by the demonstrated approach can guide the design of treatment schedules, when the projected responses of initial regimens are predicted to be ineffective.
For further reading:
Agur Z, & Vuk Pavlovic S. Personalizing immunotherapy: Balancing predictability and precision. Oncoimmunology (2012), 1(8) pp.1-3
Agur Z, and Vuk Pavlovic, S. Mathematical Modeling in Immunotherapy of Cancer: Personalizing Clinical Trials. J. Molecular Therapy (2012) 20(1) pp. 1-2.
Kogan Y., Halevi-Tobias K., Elishmereni M., Vuk-Pavlovic S., Agur Z. (2010). Development of an algorithm for in-treatment personalization of immunotherapy. To be submitted.
Kronik N., Kogan Y., Elishmereni M., Halevi-Tobias K., Vuk-Pavlovic S., Agur Z.(2010). Personalized mathematical models for prostate cancer immunotherapy can predict results of Phase IIa clinical trial., To be submitted.
Michael, A., Ball, G., Quatan, N., Wushishi, F., Russell, N., Whelan, J., et al. (2005). Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res, 11(12), 4469-4478.

