Cytokine
Background
Interleukin (IL)-21, a cytokine that was discovered a decade ago, is capable of eliciting potent antitumor responses in several solid cancers, as evidenced by an array of preclinical and clinical studies. Yet, the drug possesses complex immunomodulatory functions, and has been only partially effective in the clinic thus far. Conditions for beneficial IL-21 immunotherapy remain elusive, and a rational and systematic analysis of beneficial IL-21-based treatment strategies has long been called for.
Methods and Results
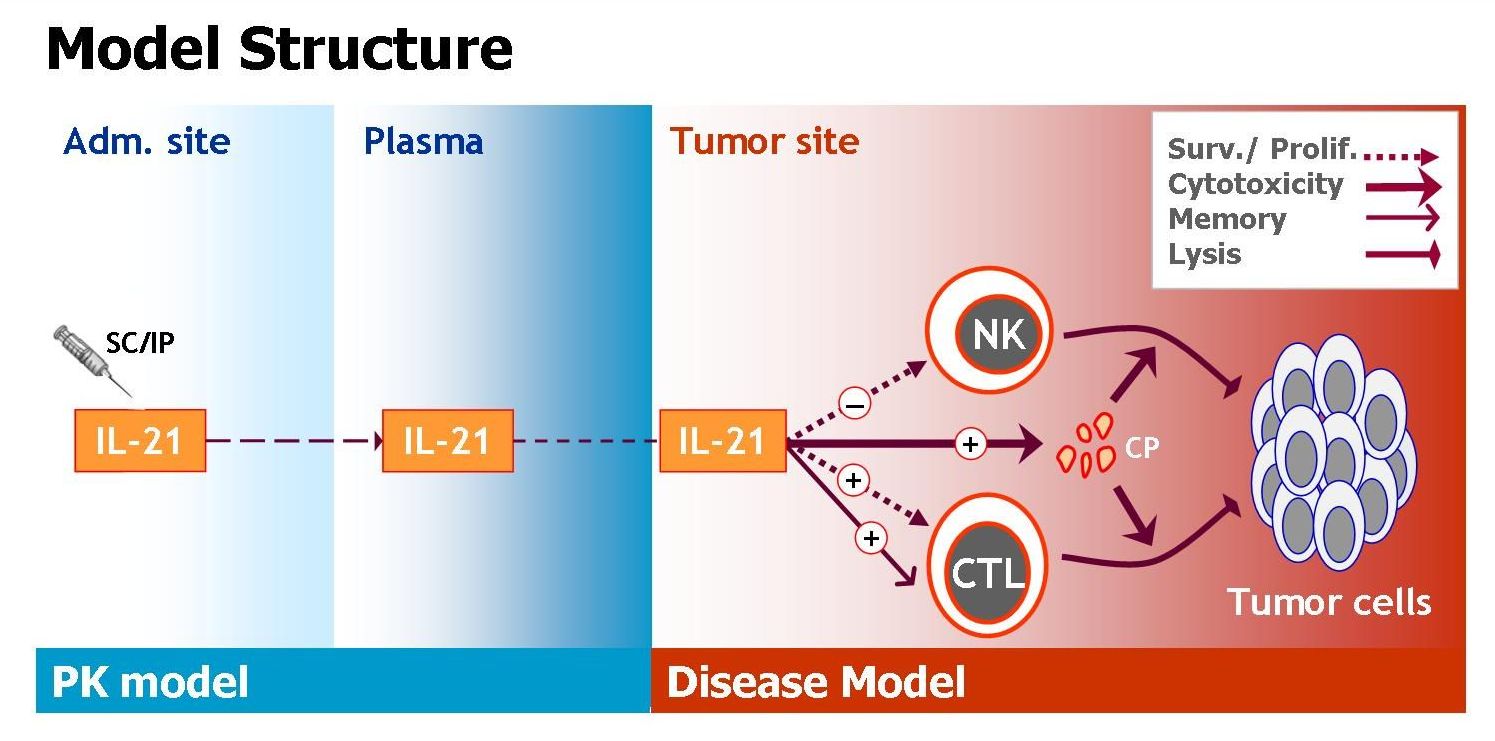
We have extensively studied the effects of IL-21 on tumor eradication, by mathematically modeling the influence of the cytokine on natural killer (NK) cell- and CD8+ T-cell-mediated lysis of solid tumor cells. The model (schemed in Figure 1) accounts for IL-21 mediated death/proliferation of cells, as well as their tumor killing capacities and memory effects. To allow for analysis of IL-21 systemic therapy, the model contains an additional pharmacokinetic (PK) component describing IL-21 bioavailability in the cancer tissue. Model parameters were estimated based on a variety of experimental studies: Data was obtained from studies in which tumor-bearing mice were treated with IL-21 via genetically-modified IL-21-secreting cells (e.g. cytokine gene therapy), and from experiments in which mice challenged with melanoma or renal cell carcinoma were treated with IL-21 systemically. The model was validated by its accurate retrieval of experimental melanoma and renal cell carcinoma dynamics under numerous IL-21 regimens using different treatment periods, doses and administration routes (Figure 2). Most parameters were independent of the tumor type or treatment design, supporting the model’s generality and relevance for various cancer indications and therapeutic conditions.
When simulating the model under several alternative regimens and strategies, we arrived at important therapeutic insights concerning systemic IL-21 therapy. First, the importance of early treatment onsets, and the benefit of reduced dose-intensities, was clearly shown in our analysis. Moreover, when studying fractionated scheduling (Figure 2), the model predicted that splitting the standard daily 50 μg regimen into an equally intense schedule of two 25 μg doses applied twice a day would result in a significantly stronger antitumor response (a 45%-reduced tumor mass). The benefit of this fractionated regimen was experimentally confirmed in melanoma-bearing mice treated by the model-suggested splitting protocol (Figure 2). This serves as additional prospective validation of the model.
Conclusions
In summary, our analysis shows that by employing mathematical methods to construct a general biologically-relevant model, IL-21 treatment strategies with improved efficacy can be fashioned. Such a tool could be highly instrumental in both streamlining and clinically applying the cytokine in solid cancer patients in the very near future.
For further reading
- Cappuccio, A., Elishmereni, M., & Agur, Z. (2006). Cancer immunotherapy by interleukin-21: potential treatment strategies evaluated in a mathematical model. Cancer Res, 66(14), 7293-7300.
- Cappuccio, A., Elishmereni, M., & Agur, Z. (2007). Optimization of interleukin-21 immunotherapeutic strategies. J Theor Biol, 248(2), 259-266
- Elishmereni, M., Kheifetz, Y., S∅ndergaard, H., Cappuccio, A., Peled, U., Overgaard, RV., Agur, Z. An in vivo validated systemic mathematical model of IL-21 immunotherapy in solid cancer for clinical design of beneficial treatment regimens. In preparation.

