Sepsis
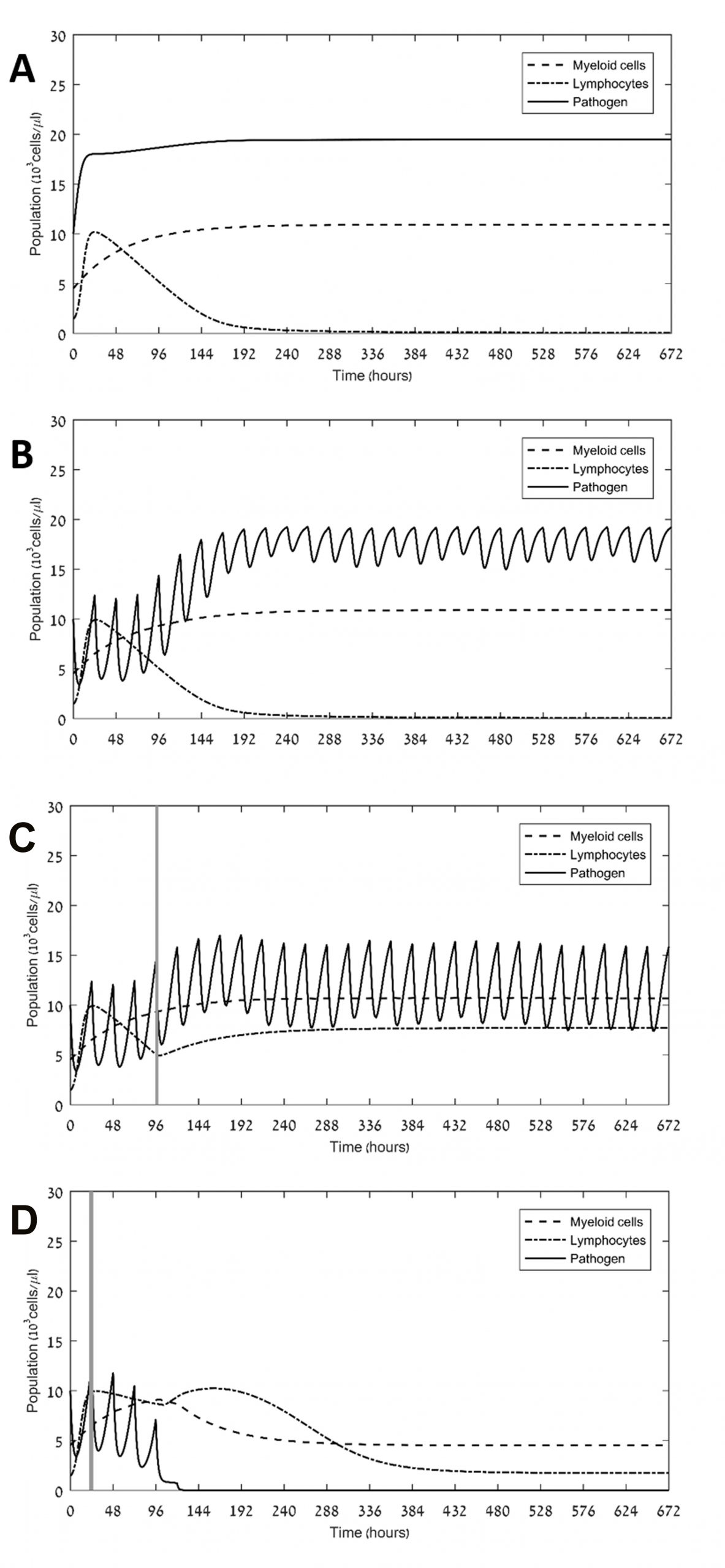
Sepsis-associated immune dysregulation, involving hyper-inflammation and immunosuppression, is a prominent problem among intensive care unit patients, often leading to multiple organ dysfunction and death. Our aim was to identify the main driving force underlying immunosuppression in sepsis, and to suggest new therapeutic avenues for controlling this immune impairment and alleviating excessive pathogen load. We developed two minimalistic (skeletal) mathematical models of pathogen-associated inflammation, which focus on the dynamics of myeloid, lymphocyte and pathogen numbers in blood. Both models rely on the assumption that the presence of the pathogen causes a bias in hematopoietic stem cell differentiation towards the myeloid developmental line. However, only in one of the models we assumed, in addition, that continuous exposure to pathogen induces lymphocyte exhaustion. We also created therapy models, both by antibiotics and immunotherapy with PD-1/PD-L1 checkpoint inhibitors. Assuming realistic parameter ranges, we simulated the pathogen-associated inflammation models in silico with or without various antibiotic and immunotherapy schedules. Computer simulations of the two models showed that the assumption of lymphocyte exhaustion is a prerequisite for attaining immunosuppression and sepsis, and that the ability of the innate and adaptive immune system to control infections depends on the pathogen’s replication rate. Simulation results further show that coupling antibiotics and immune checkpoint blockers can suffice for defeating even an aggressive pathogen within one month. This is so as long as the drugs are administered soon after diagnosis. In contrast, when applied alone antibiotics or immune checkpoint blockers fall short of eliminating aggressive pathogens in reasonable time. Our results suggest that lymphocyte exhaustion crucially drives immunosuppression in sepsis, and that one can efficiently resolve both immunosuppression and pathogenesis by timely coupling antibiotics with an immune checkpoint blocker, but not by either one of these two treatment modalities alone. Our model can be adapted to explore the potential of other therapeutic options in this field.
Gillis A, Beil M, Halevi-Tobias K, van Heerden PV, Sviri S, Agur Z. Alleviation of exhaustion-induced immunosuppression and sepsis by immune checkpoint blockers sequentially administered with antibiotics—analysis of a new mathematical model. J Intensive Care Med. Experimental. 2019 7(1):32. doi: 10.1186/s40635-019-0260-3.
Currently, we aim to optimize the therapy of patients with sepsis, admitted to the intensive care units, by use of immune checkpoint blockers (ICBs). To this end we employ methodologies from operations research [1] on a core of our previous model for sepsis [2]. Thus, pharmacokinetics and pharmacodynamics models of ertapenem and nivolumab were incorporated into the host-disease model for simulation of various drug schedules. A small therapeutic window for ICBs was found, within which immunosuppression was alleviated and the pathogen was eliminated. We concluded that efficacy of ICB administration stands to benefit greatly from modeling, in terms of finding optimal dose and timing. Our results are illustrated n the figures below.
1. Agur Z, Hassin R, Levy S. Optimizing Chemotherapy Scheduling Using Local Search Heuristics. Operations Research. 2006;54:829-46. doi: 10.1287/opre.1060.0320.
2. Gillis A, Beil M, Halevi-Tobias K, van Heerden PV, Sviri S, Agur Z. Alleviation of exhaustion-induced immunosuppression and sepsis by immune checkpoint blockers sequentially administered with antibiotics-analysis of a new mathematical model. Intensive Care Med Exp. 2019;7(1):32. doi: 10.1186/s40635-019-0260-3. PubMed PMID: 31187301; PubMed Central PMCID: PMCPMC6560115.